Eight-Factor Analysis of Kratom – 2022 School of Pharmacy, Concordia University Wisconsin
Eight-Factor Analysis of Kratom
Final Report from PHAR 537 – Medicinal Natural Products
Claudia Betancourt-Perez, Mackenzie E. Burns, Mitchell J. Glodoski, Timothy H. Vogt Uvidelio Castillo (Course Coordinator), Terry-Elinor R. Reid, Christopher W. Cunningham
Abstract. As a semester-long course project, the third-year pharmacy students of PHAR 537 (Medicinal Natural Products) completed an “eight-factor analysis” of Mitragyna speciosa (“kratom”). The eight factors are considered as part of a process by which legislatures determine whether a product should be regulated as a controlled substance. We evaluated the literature concerning the pharmacokinetic and pharmacodynamic (PK/PD) properties of M. speciosa, and its impact on public health to the United States at large and Wisconsin specifically. Based on our review of the available literature, we conclude that regulation of M. speciosa in Wisconsin as a schedule-I substance is not justified at this time. We base this conclusion, in part, on the scientific evidence demonstrating that M. speciosa and its chemical constituents have lower potential for overdose and abuse relative to other agents that are not scheduled in this way. We believe that controlling M. speciosa and its chemical constituents under schedule-I harms public health and stifles much-needed research into its therapeutic and toxic properties.
I. Introduction
Per Wisconsin statute 961 (Uniform Controlled Substances Act), the state legislature has the authority to regulate the “manufacture, distribution, delivery, possession, and use of controlled substances for other than legitimate purposes” [1]. The authority to determine whether a substance shall be scheduled is given to the Controlled Substances Board (CSB) [2], and the CSB shall consider the following factors, generally known as the “eight factors” [3]:
|
(a) The actual or relative potential for abuse; |
|
(b) The scientific evidence of its pharmacological effect, if known; |
|
(c) The state of current scientific knowledge regarding the substance; (d) The history and current pattern of abuse; |
|
(e) The scope, duration and significance of abuse; |
|
(f) The risk to the public health; |
|
(g) The potential of the substance to produce psychological or physical dependence liability; and |
|
(h) Whether the substance is an immediate precursor of a substance already controlled under this chapter. |
Further, the CSB “shall add a substance to schedule I upon finding that the substance:
(a) Has high potential for abuse;
(b) Has no currently accepted medical use in treatment in the United States;
(c) Lacks the accepted safety for use in treatment under medical supervision.” [4]
Alternately, the CSB could schedule a substance to schedule I if it is controlled in this way under 21 USC 812 (c) [5].
Controlling a substance under schedule I has broad consequences. First, there are legal consequences to individuals who are caught with a compound that is controlled under schedule-I, as the penalties for possessing schedule-I compounds are generally harsher than those for compounds that are regulated under higher schedules [6]. Patients that experience legitimate therapeutic value from products that are regulated under schedule-I would also be harmed, as scheduling substances in this way effectively prevents them from accessing the therapeutic agent. The process of controlling substances also has consequences for research and innovation. In terms of research, schedule-I substances are subject to stricter control and regulation, which adversely impacts the ability of faculty at smaller schools to engage in scholarship related to these substances [7]. Such scheduling also adversely impacts innovation: businesses seeking to develop
1
Eight-Factor Analysis of Kratom – 2022 School of Pharmacy, Concordia University Wisconsin
medicinal products would be disincentivized from working with partners in states that label products as schedule-I substances [7].
Recognizing the significance that scheduling a substance has on patient health and beyond, our class took on the challenge of conducting an “eight-factor analysis” of Mitragyna speciosa, also known as “kratom.” Two constituents of M. speciosa, termed mitragynine (MG) and 7-hydroxy-mitragynine (7-OH-MG), are explicitly listed under schedule-I in the state of Wisconsin [8]. This project was conducted as a part of a 3rd year elective course for Pharm.D. graduate students at Concordia University Wisconsin called Medicinal Natural Products (PHAR 537). What follows is the result of our independent review of the available literature surrounding this medicinal plant. In the next section, we will summarize our findings in the context of the “eight factors” outlined above. Of note, none of the students of PHAR 537, nor the instructional faculty, have conducted research using M. speciosa, its constituents, or their derivatives, nor do any of the co-authors of this document have plans to do so in the immediate future. This project is an exercise in state and federal pharmacy law, and we intend for this analysis to be potentially of value to the Wisconsin CSB as they consider whether schedule-I is the appropriate place for the constituents of kratom.
II. ResultsandDiscussion
Aiding our research were two recent reviews that were written by experts in the field of substance use disorders [9][10]. These two articles provided helpful content and context as we conducted this analysis. Since the second article was written in 2021, we also sought to find newer articles that were published in 2022 that could further aid the discussion. Our analysis can be considered complementary to these articles previously published; we agree with their assessment that kratom should not be considered a controlled substance at this time.
a. Factor 1: The actual or relative potential for abuse. For this factor, we considered behavioral tests in animals performed using kratom or its purified constituents (MG, 7-OH-MG).
The first test we considered under this factor was the intracranial self-stimulation (ICSS) test. In this test, an animal is placed in a chamber and will receive electrical stimulation when it presses a lever. The first ICSS test we reviewed was conducted in 2020 by Behnood-Rod, et al [11]. In this procedure, a dose of drug is considered rewarding if it decreases brain reward threshold and is considered aversive if it increases the brain reward threshold. At low doses, MG slightly lowered the reward threshold and at high doses, MG slightly increased reward threshold, indicating that there is a mild dose-dependent rewarding effect. 7-OH-MG slightly lowered reward threshold at lower doses, but significantly increased the reward threshold at higher doses, indicating that there is a strong aversive effect of 7-OH-MG at high doses. When compared to morphine, the effects of MG and 7-OH-MG are less rewarding.
The drug self-administration (SA) test determines whether an animal will work to receive a dose of drug. Under this paradigm, a drug that has high potential for abuse will be readily self-administered by an animal, and a drug that has low abuse liability will not. The first SA test we reviewed was conducted by Hemby, et al., in 2018 [12]. This test was set up to first train rats to self-administer morphine, then determine whether those rats would instead self-administer MG or 7- OH-MG. In this test, only 7-OH-MG substituted for morphine. After the rats were substituted to MG or 7-OH-MG, the rats that substituted with MG showed a significant decrease in morphine self-administration and those that received 7-OH- MG showed a significant increase in morphine self-administration. Major conclusions from this study were: 1) that MG does not show abuse liability; 2) that because MG significantly decreased morphine self-administration, MG is potentially therapeutically valuable as a treatment for opioid abuse; and 3) that 7-OH-MG has abuse liability. A second SA test that was published by Yue, et al. [13], also showed that MG has low abuse liability and decreases self-administration of heroin.
The conditioned place preference (CPP) test determines whether an animal spends more time in a drug-paired chamber (rewarding behavior) or less time in the drug-paired chamber (aversive behavior). Yussof, et al. [14] showed that MG produced CPP at doses of 10 and 30 mg/kg following injection, which was similar to morphine. Unlike morphine,
2
Eight-Factor Analysis of Kratom – 2022 School of Pharmacy, Concordia University Wisconsin
however, MG produced anxiolytic effects at low and high doses. A similar U-shaped dose-response curve was observed for locomotor behavior, with MG stimulating locomotion at low and high doses. The authors concluded that MG has abuse liability and can produce effects that are similar to those of psychostimulant and opiate drugs. Similar conclusions were drawn by Iman, et al. [15] and Japarin, et al. [16], though it should be noted that the rewarding effects of MG were observed when MG was administered at higher doses (10-30 mg/kg, ip).
In 2019, Meepon and Sooksawate [17] reported that MG at doses from 30-90 mg/kg (ip) induced preference for the drug-paired chamber in rats; however, at doses from 10-30 mg/kg, MG significantly blocked morphine CPP, suggesting that the rewarding effects of morphine could be attenuated by MG. MG at doses between 10-30 mg/kg (ip) also blocked naloxone-precipitated withdrawal from chronic morphine, again suggesting that MG holds promise as a potential treatment option for patients experiencing opioid withdrawal.
b. Factor 2: The scientific evidence of its pharmacological effect. For this factor, we reviewed additional behavioral tests that demonstrate that M. speciosa alkaloids have pharmacologic activity in vivo. The in vivo tests described above would also be considered evidence that MG and 7-OH-MG produce a pharmacologic effect in subjects.
The first test we considered was the drug discrimination (DD) test. In this test, an animal is trained to respond to the stimulus effects of a training drug and then compare whether the animal responds in a similar way to a test drug, in this case MG or 7-OH-MG. The DD test can be useful for determining whether a test drug works through a similar mechanism of action as a training drug.
The first DD test we reviewed was published in 2015 [18]. In this study, a two-lever DD test was used to see if male rats could discriminate MG from vehicle and whether MG would substitute for morphine in rats trained to discriminate morphine. The ability of rats to discriminate morphine from vehicle was also used as a comparator. This study found that MG discrimination in one group of rats was similar to morphine discrimination in a second group. Administration of MG resulted in full substitution for morphine. The authors concluded that the pharmacologic effects of morphine and MG are similar, and that MG appears to be responsible for the potential for kratom to be abused.
A second DD test [19] was published in 2019 and used male and female rats. In this study, the authors tested the ability of morphine and MG to disrupt operant responding for food and increase antinocicetption response to a thermal stimulus in the hot plate test. To determine whether the pharmacologic effects of MG were mediated by opioid receptors, the study included co-administration tests for MG with 1) the mu opioid receptor antagonist, naloxone, and 2) morphine. The results found that both MG and morphine decreased schedule-controlled responding and increased thermal antinociception, though MG was less potent than morphine. Naloxone did not block the effects of MG, suggesting a non- opioid mechanism of action for MG. The results of this study support that MG is effective in reducing pain stimuli, though the mechanism of action differs substantially from that of morphine.
c. Factor 3: The state of current scientific knowledge regarding the substance. For this factor, we considered in vitro receptor binding and efficacy studies. We also reviewed experiments that included human volunteers.
To determine the receptor binding profile of MG and 7-OH-MG, we first consulted the Ki Database provided by the Psychoactive Drug Screening Program (PDSP), which is housed at the University of North Carolina in Chapel Hill and supported as a free service by the National Institute of Mental Health (NIMH) [20]. The available binding data for MG is included in an accompanying spreadsheet. Among the opioid receptors, MG has highest affinity for mu (average MOR Ki 624.2 nM), then kappa (average KOR Ki 823.25 nM), then delta (DOR Ki 2637 nM). MG also has weak (micromolar) affinity for certain serotonin receptors (5-HT1A, 5-HT1D, 5-HT2B, 5-HT7), adrenergic receptors (alpha2A, alpha2B, alpha2C), and dopamine receptors (D2). For comparison, morphine has nM affinity for opioid receptors (MOR ~ KOR > DOR) and negligible affinity for other monoamine receptors.
Two papers described opioid receptor binding and efficacy of MG and 7-OH-MG in detailed functional assays [21][22]. In these experiments, researchers determined the functional selectivity (aka signaling bias) of kratom alkaloids 3
Eight-Factor Analysis of Kratom – 2022 School of Pharmacy, Concordia University Wisconsin
for activating G protein pathways or beta-arrestin pathways. Both studies found that MG and 7-OH-MG were G protein- biased partial agonists of MOR, KOR, and DOR, and neither recruited arrestins. In contrast, morphine is a non-biased MOR agonist; this distinction in PD profile is important, as beta-arrestin2 is associated with respiratory depression and constipation, two key adverse effects of MOR agonists [23].
A 2022 study published by Henningfield, et al., compared the respiratory depressant effects of oral MG (20-400 mg/kg, po) to oral oxycodone (6.75-150 mg/kg, po) in rats [24]. Whereas oxycodone produced significant, dose- dependent sedative and respiratory depressant effects, MG produced mild sedative effects at the highest doses and no respiratory depressant effects at any doses, demonstrating the significant different in observed pharmacologic profiles between canonical MOR agonists and kratom alkaloids.
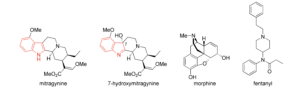
Structurally, MG and 7-OH-MG are unrelated to other natural and synthetic MOR agonists (Figure 1). MG and 7- OH-MG are considered indole alkaloids, whereas morphine (a natural MOR agonist) is considered a phenanthrene derivative and fentanyl (synthetic MOR agonist) is a 4-anilidopiperidine. All of these MOR agonists share in common a basic amine group, thus they are all alkaloids. There are over 40 indole alkaloids present in the plant that have been reported to date. 7-OH-MG is present in the leaves of M. speciosa, though in quantities that are unlikely to contribute to its pharmacologic effect when taken orally; however, MG is metabolized into 7-OH-MG in vivo, and could indeed be considered an active metabolite of oral MG. More research is needed to determine this.
Figure 1. Structures of mitragynine (MG), 7-hydroxymitragynine (7-OH-MG), morphine (a naturally occurring MOR agonist), and fentanyl (a synthetic MOR agonist). The indole group of MG and 7-OH-MG is shown in red.
The PK profile of MG was determined in healthy male volunteers who were regular users of kratom [25]. When administered orally as a tea, the terminal half-life (t1/2) was 23.24 ± 16.07 h, the time to Tmax was 0.83 ± 0.35 h, volume of distribution (Vd/F) was 38.04 ± 24.32 L/kg, and the clearance (CL/F) was 98.1 ± 51.34 L/h kg. In 2022, Tanna, et al., published the results of a clinical PK study using a single low (2g) dose of kratom orally to six healthy volunteers [26]. This study found the following parameters using a two-compartment model: t1/2, 1.76 ± 0.0163 h, Tmax 1.13 ± 0.111 h, V1/F 1170 ± 105 L, CL/F 227 ± 8.11 L/h. In contrast to the earlier study, this study used standardized kratom material that had thoroughly characterized alkaloid content.
According to Smith, et al., the median typical dose of kratom by frequent users was reported to be 4.57 ± 3.61 g, and the median number of doses per day was 2.68 ± 1.73 [27]. The median age of kratom use initiation (29.9 ± 8.8 y) was higher than for initiation of alcohol (15 ± 3.3 y), nicotine (15.9 ± 4.5 y), and cannabis (16.8 ± 5.4 y). Ya, et al., reported that the median oral bioavailability of MG is approximately 21% [28].
d. Factor 4: The history and current pattern of abuse. Kratom has been used traditionally in Southeast Asia, the Philippines, and New Guinea. Traditionally, the leaves (dried or fresh) are chewed or brewed into a tea. Kratom leaves are
4
Eight-Factor Analysis of Kratom – 2022 School of Pharmacy, Concordia University Wisconsin
used in this way to battle physical fatigue, improve mood, relieve pain, and help treat opiate addiction [29][30]. Use of kratom is restricted or banned in most of Europe, Indonesia, Argentina, Israel, New Zealand, and Australia [31]. In the United States, kratom is illegal to buy, sell, possess and use in 6 states: Alabama, Arkansas, Indiana, Rhode Island, Vermont, and Wisconsin [32]. Though the US Drug Enforcement Agency (DEA) initially proposed to control the use of kratom under its emergency scheduling authority in August 2016, this was withdrawn months later in October 2016 [33]. It is difficult to find reputable data regarding the current pattern of abuse of kratom in Wisconsin.
e. Factors 5 and 6: The scope, duration, and history of abuse, and the risk to public health. 0.8% of people over the age of 12 in the United States (2.1 million people) used kratom in 2020 [34]. For comparison, 17.9% (49.6 million) used cannabis in the past year, and 3.4% (9.5 million) misused opioids in the same period. Kratom use was lowest among younger people (adolescents age 12-17, 0.2%). According to the 2020 Annual Report of the American Association of Poison Control Centers [35], there were 1262 calls to poison control centers regarding kratom. For context, there were 10,636 calls regarding the FDA-approved cardiovascular drug clonidine, and 17,051 concerning the OTC antihistamine cetirizine (generic for Zyrtec®). Table 1 compares poison center calls for kratom compared to heroin, prescription fentanyl products, and methadone, which is an FDA-approved treatment for opioid use disorder (OUD). When compared to these products, there were fewer calls made regarding kratom, and the incidence of major outcomes or death were also reduced. Notably, these data for kratom are an improvement over methadone. A 2022 report using data from the British Columbia Drug and Poison Information Centre in Canada found that there were 32 calls regarding kratom between 2012-2019, at increasing frequency near the end of the study period; there were no deaths and the authors attributed the increase potentially to more patients with opioid use disorder (OUD) using kratom to manage their disease [36].
Table 1. Calls to US poison centers regarding single substance pharmaceutical exposures to kratom, heroin, prescription fentanyl, and methadone, and selected outcomes. Data from ref [35].
There were 152 unintentional overdose deaths between July 2016 and December 2017 that tested positive for kratom [37]. Of those, in only 7 (4.6%) did the deceased test positively for kratom only. In this period, there were 27,338 drug overdose deaths, meaning kratom was detected in 0.56% of them. Of the polydrug deaths involving kratom, 65% of postmortem samples tested positively for fentanyl, 33% tested positively for heroin (as metabolites), and nearly 20% tested positively for prescription opioids. At doses over 25 g, patients are at risk of hospitalization due to respiratory depression, hallucinations, seizures, and psychosis [38].
A 2022 study investigated the impact of the covid-19 pandemic on kratom use in comparison to use of other drugs of abuse [39]. This study found that there 33% reported an increase in kratom use compared to the period before the pandemic and 24% reported a decrease in use. Alcohol, tobacco, and prescription opioid use were all more likely to have gotten worse during the pandemic. A 2022 study found that reasons for using kratom are diversifying, with users indicating that they are using the product as, among other things, a treatment substitute for opioids, alcohol, and stimulants [40].
Adverse effects of kratom include: loss of muscle coordination; constipation; dizziness; hypotension; increased alertness; and tachycardia. These adverse effects can vary in severity based on the amount and strain of product consumed. In one case report, a 15 year old Caucasian female presented to the emergency department after consuming 45 capsules of kratom 500 mg (22.5 g) in a suicide attempt [41]. Notably, the patient did not show signs of respiratory depression or loss of consciousness, which are hallmarks of the opioid toxidrome and could be life-threatening. Another
5
Eight-Factor Analysis of Kratom – 2022 School of Pharmacy, Concordia University Wisconsin
case report concerned a 37 year old Caucasian male who presented to the emergency department unresponsive, with minimal response to naloxone [42]. The patient’s family reported that he consumed 500 g of kratom the previous day.
f. Factor 7: The potential of the substance to produce psychological or physical dependence liability. An individual is considered physically dependent on a substance if they experience withdrawal symptoms when drug use is abruptly ceased. In addition to the studies discussed above, we also reviewed investigations into kratom withdrawal and how kratom impacts withdrawal from other drugs of abuse.
Wilson, et al. [43] determined physical dependence using an induced hyperalgesia model in mice. Products tested include a kratom alkaloid extract (KAE) and MG, both administered orally. Induction of hyperalgesia was used as a marker for drug dependence. Additionally, the team investigated naloxone-precipitated withdrawal following chronic opioid treatment. Like morphine, KAE and MG produced hyperalgesia after 5 days. Following naloxone administration, the somatic signs of withdrawal were strongest with morphine and attenuated in mice dependent on KAE and MG. Furthermore, mice administered KAE or MG demonstrated fewer withdrawal signs than mice who continued to receive morphine. These results suggest 1) that KAE or MG has lower dependence liability than morphine, and 2) that KAE or MG could be useful as treatments for opioid withdrawal. A cross-sectional study conducted in Thailand found that users were likely to experience signs of physical dependence that were directly related to duration, frequency, and amount of kratom consumed [44]. As mentioned above, Gutridge, et al. [21] showed that kratom alkaloids were effective in reducing ethanol intake in mice, suggesting that kratom may have therapeutic potential in patients with alcohol use disorder (AUD).
g. Factor 8: Whether the substance is an immediate precursor of a substance already controlled under this chapter. As mentioned in section II.c and shown in Figure 1, MG and 7-OH-MG are structurally unrelated to other opioids and to other agents under strict control in Wisconsin.
III. Conclusions
Kratom is a plant-based product that has a long history of traditional use in Southeast Asia and recently has gained attention in the United States as both a recreational substance and an herbal treatment for drug and alcohol use disorders. Though the subjective and pharmacologic effects are similar to MOR agonists like morphine and fentanyl, the indole alkaloids present in M. speciosa are structurally and pharmacodynamically distinct.
Several points must be addressed when considering the translation of animal studies to the human condition. First, animal studies using purified MG and/or 7-OH-MG will not necessarily tell an accurate story of the pharmacologic profile of the M. speciosa plant material because other constituents of the plant – e.g., other minor indole alkaloids, terpenes, flavonoids, etc. – could influence MG or 7-OH-MG PK. This is commonly observed with natural products-based pharmacologic research. Second, many in vivo studies using purified alkaloids administer those compounds via injection (e.g., ip), which is not the way kratom is typically consumed [26].
It is important to consider polydrug abuse when reading drug overdose statistics. For example, Olsen, et al. [37] reported that nearly 2/3 of all drug overdose deaths in 2016-2017 involving kratom also tested positively for fentanyl, a high-potency/high-efficacy MOR agonist that, depending on dose, can have minimal response to naloxone [45]. A 2022 report [46] described kratom products that were adulterated with other high-potency MOR agonists, highlighting the need for detailed analysis of kratom products when asking the question, “what is to blame for this overdose?”
The limited number of case reports and national overdose deaths suggests that the risks of kratom are low. Nonetheless, the few case reports that are available require critical examination. For example, in ref [41], the patient consumed a quantity that is over 5x the typical psychoactive dose in a suicide attempt. An even higher dose was observed in ref [42]. Other issues associated with the interpretation of case reports were recently raised by Smith, et al. [47]. It has been understood since Paracelsus that “the dose makes the remedy or the poison,” and so labeling a substance as “toxic” based on a small number of case reports where the dose is high seems excessive.
6
Eight-Factor Analysis of Kratom – 2022 School of Pharmacy, Concordia University Wisconsin
As a final consideration, an early eight-factor analysis of kratom [9] reported that “abrupt discontinuation [of kratom use] may be accompanied by withdrawal symptoms that are qualitatively similar but generally weaker than those observed following discontinuation of opioids.” This was updated in 2021: “Some user reports suggest that regular kratom consumption carries risks of dependency and addiction, though with generally self-manageable withdrawal” [48]. The key phrase “self-manageable withdrawal” distinguishes kratom from other opioid agonists that have a severe withdrawal profile.
In conclusion, kratom is a plant product that produces subjective effects distinct from those of other opioids that have high abuse liability. The risk of life-threatening respiratory depressant effects appears to be very low, again different from MOR agonists with high risk of overdose like heroin and fentanyl. Though calls to poison centers in the United States and Canada appear to be increasing, the number of calls is low compared to other, high-risk drugs and may be due to self- medication as part of the ongoing opioid public health crisis. Preclinical assessment of kratom and its constituents suggest that the risk of dependence and withdrawal is minor compared to other drugs that are considered controlled substances, and that kratom and its alkaloid constituents may be therapeutically useful as treatments for substance use disorders when used under the supervision of a clinician. Finally, the US DEA and legislatures of 44 of 49 other US states do not believe that kratom or its constituents meet the requirements to be a schedule-I controlled substance. We agree.
IV. References
- [1] WI 961.001(1m). https://docs.legis.wisconsin.gov/statutes/statutes/961. Accessed 26 October, 2022.
- [2] WI 961.11(1). https://docs.legis.wisconsin.gov/statutes/statutes/961. Accessed 26 October, 2022.
- [3] WI 961.11(1m)(a-h). https://docs.legis.wisconsin.gov/statutes/statutes/961. Accessed 26 October, 2022.
- [4] WI 961.13(1m)(a-c). https://docs.legis.wisconsin.gov/statutes/statutes/961. Accessed 26 October, 2022.
- [5] WI 961.13(2m). https://docs.legis.wisconsin.gov/statutes/statutes/961. Accessed 26 October, 2022.
- [6] WI 961, Subchapter IV: Offenses and Penalties. https://docs.legis.wisconsin.gov/statutes/statutes/961. Accessed 26 October, 2022.
- [7] Comer, S.D.; Pravettoni, M.; Coop, A.; Baumann, M.H.; Cunningham, C.W. Potential unintended consequences of class-wide drug scheduling based on chemical structure: A cautionary tale for fentanyl- related compounds. CPDD News and Views 2021. doi: 10.1016/j.drugalcdep.2021.108530.
- [8] WI 961.14(mk and mL). https://docs.legis.wisconsin.gov/statutes/statutes/961. Accessed 26 October, 2022.
- [9] Henningfield, J.E.; Fant, R.V.; Wang, D.W. The abuse potential of kratom according the 8 factors of the controlled substances act: implications for regulation and research. Psychopharmacology 2018, 235, 573- 589.
- [10] Henningfield, J.E.; Wang, D.W.; Huestis, M.A. Kratom abuse potential 2021: an updated eight factor analysis. Front. Pharmacol. 2021, 12, 775073.
- [11] Behnood-Rod, A.; Chellian, R.; Wilson, R.; Hiranita, T.; Sharma, A.; Leon, F.; McCurdy, C.R.; McMahon, L.R.; Bruijnzeel, A.W. Evaluation of the rewarding effects of mitragynine and 7- hydroxymitragynine in an intracranial self-stimulation procedure in male and female rats. Drug Alcohol Depend. 2020, 215, 108235.
- [12] Hemby, S.E.; McIntosh, S.; Leon, F.; Cutler, S.J.; McCurdy, C.R. Abuse liability and therapeutic potential of the Mitragyna speciosa (kratom) alkaloids mitragynine and 7-hydroxymitragynine. Addict. Biol. 2018, 24, 874-885.7
Eight-Factor Analysis of Kratom – 2022 School of Pharmacy, Concordia University Wisconsin
- [13] Yue, K.; Kopajtic, T.A.; Katz, J.L. Abuse liability of mitragynine assessed with a self-administration procedure in rats. Psychopharmacology 2018, 235, 2823-2829.
- [14] Yusoff, N.H.M.; Suhaimi, F.W.; Vadivelu, R.K.; Hassan, Z.; Rümler, A.; Rotter, A.; Amato, D.; Dringenberg, H.C.; Mansor, S.M.; Navaratnam, V.; Müller, C.P. Abuse potential and adverse cognitive effects of mitragynine (kratom). Addict. Biol. 2014, 21, 98-110.
- [15] Iman, I.N.; Ahmad, N.A.; Mohd Yusof, N.A.; Talib, U.N.; Norazit, A.; Kumar, J.; Mehat, M.Z.; Hassan, Z.; Müller, C.P.; Muzaimi, M. Mitragynine (kratom)-induced cognitive impairments in mice resemble delta-9-THC and morphine effects: Reversal by cannabinoid CB1 receptor antagonism. Front. Pharmacol. 2021, 12, 708055.
- [16] Japarin, R.A.; Yusoff, N.H.; Hassan, Z.; Müller, C.P.; Harun, N. Cross-reinstatement of mitragynine and morphine place preference in rats. Behav. Brain Res. 2021, 399, 113021.
- [17] Meepong, R.; Sooksawate, T. Mitragynine reduced morphine-induced conditioned place preference and withdrawal in rodents. Thai J. Pharm. Sci. 2019, 43, 21-29.
- [18] Harun, N., Hassan, Z., Navaratnam, V., Mansor, S. M., & Shoaib, M. (2015). Discriminative stimulus properties of mitragynine (kratom) in rats. Psychopharmacology, 232(13), 2227–2238.
- [19] Hiranita, T., Leon, F., Felix, J. S., Restrepo, L. F., Reeves, M. E., Pennington, A. E., Obeng, S., Avery, B. A., McCurdy, C. R., McMahon, L. R., & Wilkerson, J. L. (2019). The effects of Mitragynine and morphine on schedule-controlled responding and antinociception in rats. Psychopharmacology, 236(9), 2725–2734.
- [20] Besnard, J.; Ruda, G.F.; Setola, V.; Abecassis, K.; Rodriguiz, R.M.; Huang, X.P.; Norval, S.; Sassano, M.F.; Shin, A.I.; Webster, L.A.; Simeone, F.R.; Stojanovski, L.; Prat, A.; Seidah, N.G.; Constam, D.B.; Bickerton, G.R.; Read, K.D.; Wetsel, W.C.; Gilbert, I.H.; Roth, B.L.; Hopkins, A.L. Automated design of ligands to polypharmacological profiles. Nature. 2012, 492, 215-220.
- [21] Gutridge, A.M.; Robins, M.T.; Cassell, R.J.; Uprety, R.; Mores, K.L.; Ko, M.J.; Pasternak, G.W.; Majumdar, S.; van Rijn, R.M. G protein-biased kratom-alkaloids and synthetic carfentanil-amide opioids as potential treatments for alcohol use disorder. Br. J. Pharmacol. 177, 1497-1513.
- [22] Todd, D.A.; Kellogg, J.J.; Wallace, E.D.; Khin, M.; Flores-Bocanegra, L.; Tanna, R.S.; McIntosh, S.; Raja, H.A.; Graf, T.N.; Hemby, S.E.; Paine, M.F.; Oberlies, N.H.; Cech, N.B. Chemical composition and biological effects of kratom (Mitragyna speciosa): In vitro studies with implications for efficacy and drug interactions. Sci. Rep. 2020, 10, 19158.
- [23] Raehal, K.M.; Walker, J.K.L.; Bohn, L.M. Morphine side effects in beta-arrestin 2 knockout mice. J. Pharmacol. Exp. Ther. 2005, 314, 1195-1201.
- [24] Henningfield, J.E.; Rodricks, J.V.; Magnuson, A.M.; Huestis, M.A. Respiratory effects of oral mitragynine and oxycodone in a rodent model. Psychopharmacology (Berl.) 202, 239, 3793-3804.
- [25] Trakulsrichai, S.; Sathirakul, K.; Auparakkitanon, S.; Krongvorakul, J.; Sueajai, J.; Noumjad, N.; Sukasem, C.; Wananukul, W. Pharmacokinetics of mitragynine in man. Drug Des. Devel. Ther. 2015, 9, 2421-2429.
- [26] Tanna, R.S.; Nguyen, J.T.; Hadi, D.L.; Manwill, P.K.; Flores-Bocanegra, L.; Layton, M.E.; White, J.R.; Cech, N.B.; Oberlies, N.H.; Rettie, A.E.; Thummel, K.E.; Paine, M.F. Clinical pharmacokinetic8
Eight-Factor Analysis of Kratom – 2022 School of Pharmacy, Concordia University Wisconsin
assessment of kratom (Mitragyna speciosa), a botanical product with opioid-like effects in healthy adult participants. Pharmaceutics 2022, 14, 620.
- [27] Smith, K.E.; Rogers, J.M.; Dunn, K.E.; Grundmann, O.; McCurdy, C.R.; Schriefer, D.; Epstein, D.H. Searching for a signal: Self-reported kratom dose-effect relationships among a sample of US adults with regular kratom use histories. Front. Pharmacol. 2022, 13, 765917.
- [28] Ya, K.; Tangamornsukan, W.; Scholfield, C.N.; Methaneethorn, J.; Lohitnavy, M. Pharmacokinetics of mitragynine, a major analgesic alkaloid in kratom (Mitragyna speciosa): a systematic review. Asian J. Psychiatry 2019, 43, 73-82.
- [29] Cinosi, E.; Martinotti, G.; Simonato, P.; Singh, D.; Demetrovics, Z.; Roman-Urrestarazu, A.; Saverio Bersani, F.; Vicknasingam, B.; Piazzon, G.; Li, J.-H.; Yu, W.-J.; Kapitany-Föveny, M.; Farkas, J.; Di Giannantonio, M.; Corazza, O. Following “the roots” of kratom (Mitragyna speciosa): The evolution of an enhancer from traditional use to increase work and productivity in Southeast Asia to a recreational psychoactive drug in Western countries. Biomed. Res. Int. 2015, 968786.
- [30] Han C, Schmitt J, Gilliland KM. DARK Classics in Chemical Neuroscience: Kratom. ACS Chem Neurosci 2020;11:3870–80
- [31] https://worldpopulationreview.com/country-rankings/kratom-legality-by-country, Accessed 16 November, 2022.
- [32] Is Kratom Legal? Kratom Legality by State. Sprout Health Group. Published October 28, 2020. Accessed November 21, 2022. https://www.sprouthealthgroup.com/substances/is-kratom-legal-by-state/
- [33] https://www.uspharmacist.com/article/the-dea-changes-its-mind-on-kratom. Accessed 1 December 2022.
- [34] Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. https://www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/202 0NSDUHFFR1PDFW102121.pdf. Accessed 1 December 2022.
- [35] Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Bronstein, A.C.; Rivers, L.J.; Pham, N.P.T.; Weber, J. 2020 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin. Toxicol. 2021, 59, 1282-1501.
- [36] Reich, N.; Salvo, G.; Leong, D.; Wan, V.; Kosatsky, T. Kratom exposures managed by the British Columbia poison centre, 2012-2019: a descriptive analysis. CMAJ Open 2022, 10, E755-E761.
- [37] Olsen, E.O.; O’Donnell, J.; Mattson, C.L.; Schier, J.G.; Wilson, N. Notes from the field: unintentional drug overdose deaths with kratom detected – 27 states, July 2016-December 2017. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 326-327.
- [38] Eastlack, S.C.; Cornett, E.M.; Kaye, A.D. Kratom-Pharmacology, clinical implications, and outlook: A comprehensive review. Pain Ther. 2020 Jun;9(1):55-69.
- [39] Rogers, J.M.; Smith, K.E.; Schriefer, D.; Epstein, D.H. For better or worse: self-reported changes in kratom and other substance use as a result of the COVID-19 pandemic. Subst. Abuse 2022, 16, 11782218221123977.9
Eight-Factor Analysis of Kratom – 2022 School of Pharmacy, Concordia University Wisconsin
- [40] Smith, K.E.; Dunn, K.E.; Rogers, J.M.; Grundmann, O.; McCurdy, C.R.; Garcia-Romeu, A.; Schriefer, D.; Swogger, M.T.; Epstein, D.H. Kratom use as more than a “self-treatment.” Am. J. Drug Alcohol Abuse 2022, 1-11.
- [41] Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. International Journal of Legal Medicine. 2015;130(1):127-138.
- [42] Palasamudram Shekar S, Rojas EE, D’Angelo CC, Gillenwater SR, Martinez Galvis NP. Legally Lethal Kratom: A Herbal Supplement with Overdose Potential. Journal of Psychoactive Drugs. 2019;51(1):28-30.
- [43] Wilson, L.L.; Chakraborty, S.; Eans, S.O.; Cirino, T.J.; Stacy, H.M.; Simons, C.A.; Uprety, R.; Majumdar, S.; McLaughlin, J.P. Kratom alkaloids, natural and semi-synthetic, show less physical dependence and ameliorate opioid withdrawal. Cell. Mol. Neurobiol. 2021, 41, 1131-1143.
- [44] Saingam, D.; Assanangkornchai, S.; Geater, A.F.; Lerkiatbundit, S. Factor analytical investigation of krathom (Mitragyna speciosa Korth.) withdrawal syndrome in Thailand. J. Psychoactive Drugs 2016, 48, 76-85.
- [45] Burns, S.M.; Cunningham, C.W.; Mercer, S.L. DARK classics in chemical neuroscience: Fentanyl. ACS Chem. Neurosci. 2018. 9, 2428–2437.
- [46] LeSaint, K.T.; Yin, S.; Sharma, A.; Avery, B.A.; McCurdy, C.R.; Waksman, J.C. Acute renal insufficiency associated with consumption of hydrocodone- and morphine-adulterated kratom (Mitragyna speciosa). J. Emerg. Med. 2022, 63, e28-e30.
- [47] Smith, K.E.; Dunn, K.E.; Epstein, D.H.; Feldman, J.D.; Garcia-Romeu, A.; Grundmann, O.; Henningfield, J.E.; McCurdy, C.R.; Rogers, J.M.; Schriefer, D.; Singh, D.; Weiss, S.T. Need for clarity and context in case reports on kratom use, assessment, and intervention. Subst. Abus. 2022, 43, 1221-1224.
- [48] Grundmann, O.; Babin, J.K.; Henningfield, J.E.; Garcia-Romeu, A.; Krugel, A.C.; Prozialeck, W.C.; Raffa, R.B.; Singh, D.; Smith, K.E. Kratom use in the United States: a diverse and complex profile. Addiction 2021, 116, 202-203.